1. Lithium hydroxide monohydrate (∼56% LiOH), Extra Pure - VWR
Bevat niet: 1.7 | Resultaten tonen met:1.7
CAS: 1310-66-3 MDL: MFCD00149772 EINECS: 215-183-4

2. What mass of LiOH is required to prepare 250 mL of a 3.55 M ...
What mass of LiOH is required to prepare 250 mL of a 3.55 M solution? a. 6 g. b. 6.75 g. c. 341 g. d. 27 g. e. 21 g. Lithium Hydroxide:.
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation.
3. Lithium hydroxide monohydrate moles to volume & weight calculation
Molecular formula: LiOH ⋅ H2O ; Elements: Hydrogen (H), Lithium (Li), Oxygen (O) ; Molecular weight: 41.962 g/mol ; Molar volume: 27.789 cm³/mol ; CAS Registry ...
Convert amount of Lithium hydroxide monohydrate in moles to volume and weight using its molecular weight and density. moles to volume and weight calculator

4. Lithium hydroxide, LiOH, at elevated densities - AIP Publishing
9 jul 2014 · We discuss the high-pressure phases of crystalline lithium hydroxide, LiOH. Using first-principles calculations, and assisted by ...
We discuss the high-pressure phases of crystalline lithium hydroxide, LiOH. Using first-principles calculations, and assisted by evolutionary structure searches

5. ScienceCentral Journals
Title: Possibility of military service-regeneration of LiOH for submarines and improvement in CO2 scrubbing performance of LiOH canisters, Journal title: ...
Title: Possibility of military service-regeneration of LiOH for submarines and improvement in CO2 scrubbing performance of LiOH canisters, Journal title: 한국마린엔지니어링학회지

6. What is the molarity of a LiOH solution if 55.0mL of ... - Homework.Study.com
... 1.7 g/mL? What is the molarity of a solution that is 26.0 % by mass phosphoric acid (H_3PO_4) and that has a density of 1.155 g / ml? Calculate the molarity ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation.
7. A solution is made by mixing 1.5 g of LiOH and 23.5 mL of 1.000 M...
A solution is made by mixing 1.5 g of LiOH and 23.5 mL of 1.000 M HNO3. (b) Calculate the concentration of Li+ remaining in solution.
A solution is made by mixing 1.5 g of LiOH and 23.5 mL of 1.000 M HNO3. (b) Calculate the concentration of Li+ remaining in solution.

8. [PDF] Lithium hydroxide, LiOH, at elevated densities
9 jul 2014 · We discuss the high-pressure phases of crystalline lithium hydroxide, LiOH. ... Li(1.7)O(1.1)H(0.8) aB and plane-wave cutoff ... G. Pearson, Acc.
9. [PDF] Thermodynamic Properties of Alkali Metal Hydroxides. Part 1. Lithium ...
15 okt 2009 · culated to be AfusHO(LiOH,cr)=22.6±1.7 kJ·mol- 1 (see ... =Li(g)+OH(g), 2200 K (1 point) ... Atomic absorption flame photometry, Li(g)+HP(g)=LiOH(g).
10. Density of Lithium hydroxide in 285 units and reference information
Density of Lithium hydroxide [LiOH]. Lithium hydroxide ... Density of Lithium hydroxide g cm3 = 1.54 g/cm³ ... 1.7 × 10-6, short tn/cm³. 0, short tn/dm³. 0.05 ...
Lithium hydroxide weighs 1 540 kg/m³ (96.13906 lb/ft³), melting point, molecular formula and weight, molar volume, CAS RN, description and reference

11. lioh aqueous solution: Topics by Science.gov
The maximum adsorption capacity for PANI and Aag-PANI are 1.7 mg/g and 64.6 mg/g, respectively, at an initial concentration of 100 mg/L. The Langmuir ...
12. Revealing the effect of LiOH on forming a SEI using a Co magnetic ...
12 okt 2023 · Elazari, G. Salitra and D. Aurbach, Challenges in the development of advanced Li-ion batteries: a review, Energy Environ. Sci., 2011, ...
View PDF VersionPrevious ArticleNext Article
13. Role of TiO 2 Phase Composition Tuned by LiOH on The ... - MDPI
... LiOH samples at 159.58 mAh/g and 150.80 mAh/g, respectively. ... 1.7 V (vs. Li+ /Li), leading to the formation ... LiOH), and 42.21 J/mol (1.3 LiOH). These ...
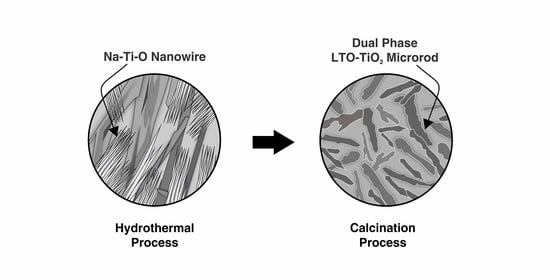
In this study, a dual-phase Li4Ti5O12-TiO2 microrod was successfully prepared using a modified hydrothermal method and calcination process. The stoichiometry of LiOH as precursor was varied at mol ratio of 0.9, 1.1, and 1.3, to obtain the appropriate phase composition between TiO2 and Li4Ti5O12. Results show that TiO2 content has an important role in increasing the specific capacity of electrodes. The refinement of X-ray diffraction patterns by Rietveld analysis confirm that increasing the LiOH stoichiometry suppresses the TiO2 phase. In the scanning electron microscopy images, the microrod morphology was formed after calcination with diameter sizes ranging from 142.34 to 260.62 nm and microrod lengths ranging from 5.03–7.37 μm. The 0.9 LiOH sample shows a prominent electrochemical performance with the largest specific capacity of 162.72 mAh/g and 98.75% retention capacity achieved at a rate capability test of 1 C. This finding can be attributed to the appropriate amount of TiO2 that induced the smaller crystallite size, and lower charge transfer resistance, enhancing the lithium-ion insertion/extraction process and faster diffusion kinetics.
14. Alkali hydroxide (LiOH, NaOH, KOH) in water: Structural and vibrational ...
3 apr 2024 · The brown line shows the total pair distribution function g(r). In the top figure, the blue and black lines, respectively, represent the ...
Structural and vibrational properties of aqueous solutions of alkali hydroxides (LiOH, NaOH, and KOH) are computed using quantum molecular dynamics simulations
